An Alberta man says he experienced a serious adverse effect from a COVID-19 vaccine and is now seeking compensation from a federal program designed to support such individuals.

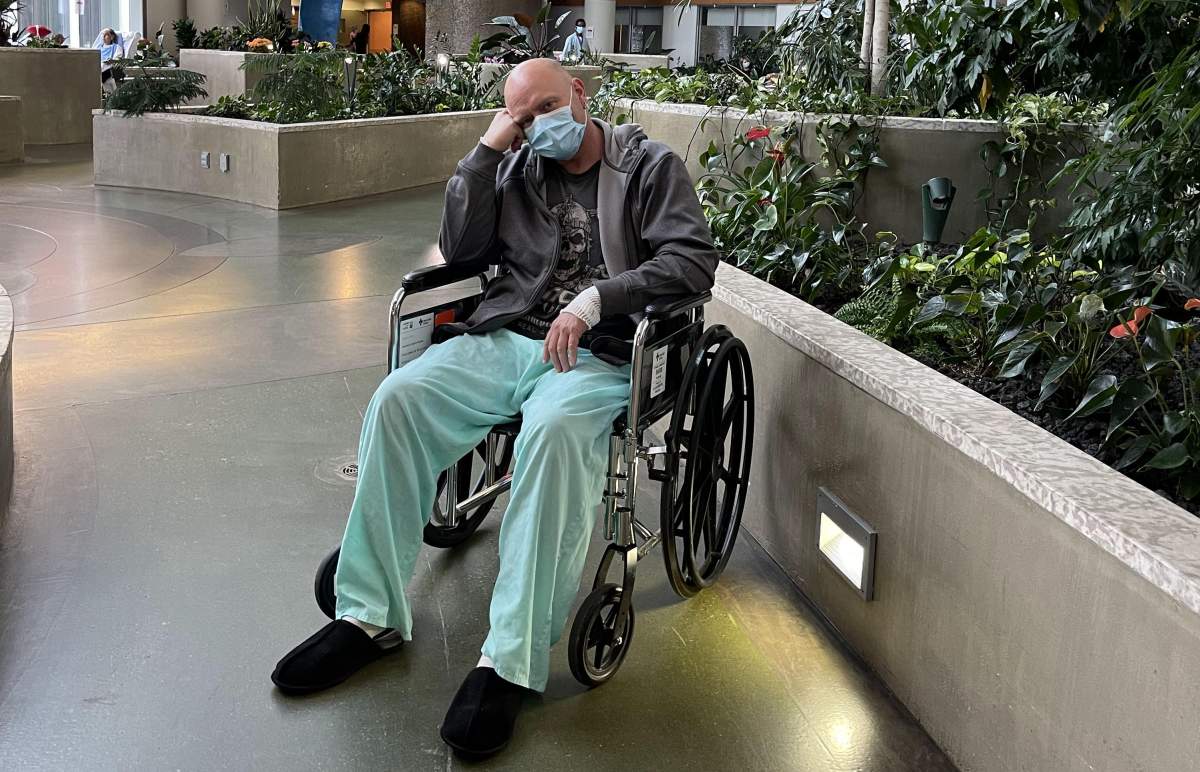
Murry Hellekson said two weeks after receiving his first dose of the AstraZeneca vaccine in April, he began experiencing tingling in his fingers and numbness on the bottom of his feet.
After going to the hospital and getting blood work done, Hellekson said he was sent home but his condition continued to decline.
“I couldn’t pick up things that I could normally grasp and pick up, and then I went to leave the work station and my left leg wouldn’t work and I went down to one knee, and then I started panicking,” he said.
READ MORE: How do you spot an adverse reaction following a COVID-19 vaccine? Experts explain
The Edson, Alta., man said after being sent home from the hospital a second time, he was eventually sent to meet with neurologists in Edmonton. That’s when an issue was discovered in his spine and he underwent an intravenous transfusion.
“The one doctor asked me straight out: ‘Did you have the AstraZeneca vaccine in the last two weeks?'” Hellekson said.
“They asked me a lot of questions like: have I had an infections, lung infections, sores in your mouth… have you had any other vaccines lately?”
Hellekson said he was diagnosed with Guillain-Barré Syndrome (GBS) — a rare neurological disorder that results in the body’s immune system mistakenly attacking part of its peripheral nervous system. GBS can range from a very mild case with brief weakness to nearly devastating paralysis.
According to Health Canada, there have been 13 reports of GBS after a COVD-19 vaccine in the country — nine after an AstraZeneca vaccine and four after a Pfizer vaccine.
READ MORE: NACI says mRNA COVID-19 vaccines should be 2nd dose after AstraZeneca shot

Get weekly health news
A doctor filed an adverse reaction report related to his AstraZeneca shot, Hellekson said.
“You don’t know what your feet are doing from the ankle down and I still have that now,” Hellekson said.

“Basically, he was slowly being paralyzed and I knew it,” Hellekson’s spouse Jenn Donovan said.
Hellekson’s condition forced him to stop working. Donovan, who owns a store in Edson, said she also had to take time away from her business to care for Hellekson.
“It affects both of us a lot to have to do that,” she said.
“What I’m looking at is having to get rid of investments to help with this,” Hellekson said.
READ MORE: Australia limits AstraZeneca COVID-19 vaccine for people over 60 years old
Hellekson discovered Canada’s Vaccine Injury Support Program (VISP). Health Canada said the program provides fair and timely financial support to those who have experienced a serious and permanent injury as a result of receiving a Health Canada-authorized vaccine, administered in Canada, on Dec. 8, 2020 or later.
While Hellekson is happy his health is now improving, he and Donovan are concerned about the requirement that an individual experienced a “serious and permanent injury” in order to receive financial support.
“It should be worded serious and/or permanent injury,” Donovan said.
“They say, ‘OK, you might have a full recovery within three months.’ Some people could lose their entire livelihood within those three months. What’s the government doing to help?” Donovan said.
“We got that shot to help protect you, to help protect our community, to help protect our elders, to protect ourselves.”

Dr. Kumanan Wilson, a professor of medicine at the University of Ottawa and a senior scientist at the Ottawa Hospital Research Institute, helped design VISP and said the program is about fairness.
“These programs are intended to be permissive and non-adversarial… to help and not create confrontation,” Wilson said.
READ MORE: What’s happening with 2nd doses of AstraZeneca COVID-19 vaccine in Alberta?
Wilson said he has been advocating for vaccine injury compensation in Canada for the past 15 years. He wrote a report on the issue 10 years ago and amid the COVID-19 vaccine rollout in Canada, he was asked to update his work.
“We found that around 15 jurisdictions around the world had vaccine injury compensation programs. Canada was the only G7 country that didn’t have one and we identified this as a gap in our vaccine policy,” he said.
Wilson believes the federal government should have created a vaccine injury compensation program before the COVID-19 vaccine rollout, even though cases of serious adverse effects are extremely rare.

“In a Phase 3 clinical trial, we can pick up about one-in-10,000 adverse event rate, and we’ve seen, unfortunately, with this vaccine rollout there are events that sometimes do occur and they can have serious consequences,” Wilson said.
“A vaccine manufacturer really isn’t liable in this situation because they have undergone best practices… that they’ve done everything right, but sometimes, unfortunately, unforeseen circumstances happen.”
Wilson said ultimately, a panel of three physicians determine which cases receive compensation.
“The process will include a review of all required and relevant medical documentation, as well as current medical evidence, to determine if there is a probable link between the injury and the vaccine,” Health Canada said in a statement.
“If there is a probable link, the medical experts will also assess the severity and duration of the injury. This information will be used to determine the types and levels of financial support awarded to the individual or their survivor(s).”
Hellekson believes his case warrants financial support.
“I’m walking unassisted now, but the hands and feet, it’s like you do better for a couple days and then it regresses for a day.”
A GoFundMe campaign has been setup to help Hellekson.







Comments
Want to discuss? Please read our Commenting Policy first.