Sabina Vohra-Miller says she feels this day has been a “long time coming.”

As a mother of a young child, Vohra-Miller was breathing a sigh of relief for all parents in Canada at the news that children under five can soon be vaccinated against COVID-19, thanks to Health Canada’s approval Thursday of Moderna’s pediatric vaccine for infants and preschoolers.
“I’m just so happy to see that parents now finally are able to offer their children some protection,” she said in an interview.
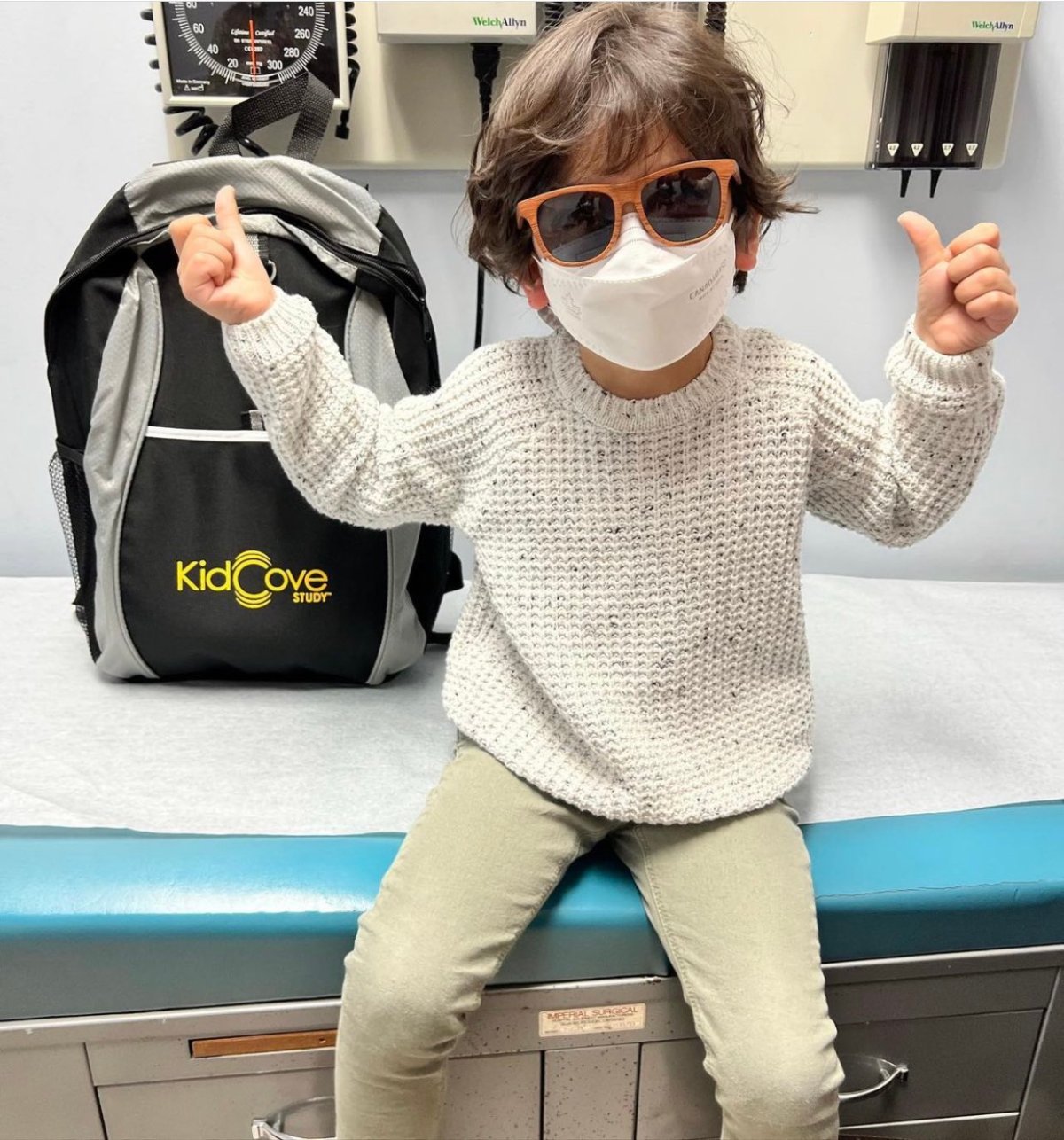
Vohra-Miller’s son was one of 500 Canadian children who took part in the clinical trials for the vaccine. Over 6,000 children in total took part in the trials.
Because her family is big into science and research, Vohra-Miller says her then-four-year-old son was excited about being part of the studies that would pave the way for more children to receive protection against COVID-19.
“He was a bit nervous, I would say, but he was also very excited about it, and in fact, he was so excited he couldn’t wait to tell all of his friends in school the next day,” Vohra-Miller said.
“It’s actually been really exciting, you know, sharing some of the science and watching his brain just expand. It’s just been fantastic.”
The Moderna Spikevax shot is the first vaccine approved for the nearly two million children under five in Canada.
- Canadian man dies during Texas Ironman event. His widow wants answers as to why
- ‘Shock and disbelief’ after Manitoba school trustee’s Indigenous comments
- Several baby products have been recalled by Health Canada. Here’s the list
- ‘Sciatica was gone’: hospital performs robot-assisted spinal surgery in Canadian first
With some provinces now experiencing a summer wave of the virus, the authorization of a vaccine for the country’s youngest is welcome news to many parents, said Canada’s deputy chief public health officer, Dr. Howard Njoo.
“A COVID-19 vaccine to protect young children has been long anticipated by many parents in Canada,” he said during a briefing Thursday.
“Today’s announcement provides parents and caregivers with a vaccine option to protect younger children in their care against COVID-19.”
The National Advisory Committee on Immunization (NACI) is recommending two doses of the vaccine “may” be offered to children six months to five years of age with dosing intervals of at least eight weeks between the first and second dose.
For children who are moderately to severely immunocompromised, NACI says the pediatric vaccine may be offered at shorter intervals of four to eight weeks between each dose.
“This is because people who are immune-compromised may have a lower immune response to COVID-19 vaccination,” Njoo said.

NACI also strongly recommends the Moderna vaccine for this age group should not be given on the same day as any other vaccines and that any non-COVID vaccines should be administered at least 14 days before or after this new COVID-19 shot.
The clinical trials of this new pediatric vaccine, which were conducted after Omicron became the dominant strain in the country, showed an immune response in children six months to five years of age that was similar to that seen in people 18 to 25 and in adults, said Dr. Supriya Sharma, chief medical advisor at Health Canada.
The vaccine was shown to be about 44 per cent effective for kids six months to two years and close to 38 per cent effective for those two to six years of age.
“As with all clinical trials, the children in the study were followed closely for any reports of side effects, and the vaccine was well-tolerated with no unexpected safety issues identified,” Sharma said.
“Most frequently reported adverse reactions for irritability or crying pain at the injection site, sleepiness, loss of appetite and fatigue. The reactions were usually mild to moderate, resolving within a few days of vaccination and no serious adverse reactions were reported.”
Patricia Gauthier, president and general manager of Moderna Canada, said while the clinical trials were performed before more highly-transmissible subvariants of the virus such as BA.5 began to circulate in Canada, the protection offered by this vaccine is still “robust” for children who, up until now, had no protection against COVID-19.
“The little ones are the ones who so far didn’t have any tools to protect themselves other than wearing a mask or washing their hands, which we know with little ones is sometimes really hard to implement,” she said.
“So now we have a new tool in the tool kit to protect the very little ones that we have.”
Hospitalization and intensive care unit (ICU) admissions among children have increased since Omicron became the predominant variant in Canada, according to data from provinces provided to the Public Health Agency of Canada (PHAC).
While severe outcomes from COVID-19 infections have been less prevalent in younger children, when hospitalizations started spiking, kids under five were the most likely to end up in hospital, said Dr. Jesse Papenburg, a pediatric infectious disease specialist and medical microbiologist at the Montreal Children’s Hospital of McGill University.
And even though Omicron and its subvariants, such as BA.5, have shown they can evade some of the immunity provided by vaccines and still infect vaccinated individuals, data from Ontario and the United States has shown that, in adolescents, the vaccine has has been over 80 per cent effective at preventing severe illness, Papenburg said.

That’s why many parents are likely happy to see this new vaccine approved for their toddlers and preschoolers, he said.
“I think there is a burden of disease there in terms of severe disease that is worth preventing with vaccination, and I think this gives us a good option to do that.”
Papenburg also noted emerging data from the 10 million children over the age five in the U.S. who have received the vaccine shows very few cases of severe side effects from the shot, including only two cases of myocarditis – an inflammation of the heart muscle – per million doses administered, Papenburg said.
“So I think that what we’re seeing now, more and more with the older children, is that the vaccine has proven to be very safe, and I would expect that to be the same for now at this younger age group,” he said.
Even so, Papenburg, Njoo and NACI are not suggesting every child must be vaccinated, but rather are recommending parents make the decision about whether to vaccinate their children on a case-by-case basis.
When making this decision, parents should be mindful that emerging data is showing that children who contract the virus, regardless of severity, can contract long COVID, Sharma said.
Njoo says he knows many parents will have questions about the safety and effectiveness of the vaccine, and he hopes they will turn to trusted sources of information for advice, such as their family doctor or a pediatrician.
“I think it’s so important that parents have to feel comfortable,” Njoo said.
“It really is a back and forth, not just a one way street in terms of giving information and then it’s up to the parent to figure it all out. So, yes, I would certainly support a good conversation.”

As for when vaccines with updated formulas targeting the Omicron variant will be available for children, it could be some time yet.
Moderna has submitted an application to Health Canada for approval of its new “bivalent” COVID-19 vaccine, designed to protect against both the original strain and Omicron, but that submission is only for those 18 and over.
However, the company is testing a children’s dose of this new formulation, Gauthier said.
“We are currently studying the bivalent vaccine in younger age populations and should have data on these younger age populations in the fall.”
In the meantime, provinces and territories, which are responsible for vaccine delivery for most populations, will begin to roll out vaccine programs for younger children soon and there will be sufficient supply for all who want one, Njoo said.





Comments