Researchers at McMaster University are set to begin human trials for a pair inhaled COVID-19 vaccines designed to combat variants of concern.

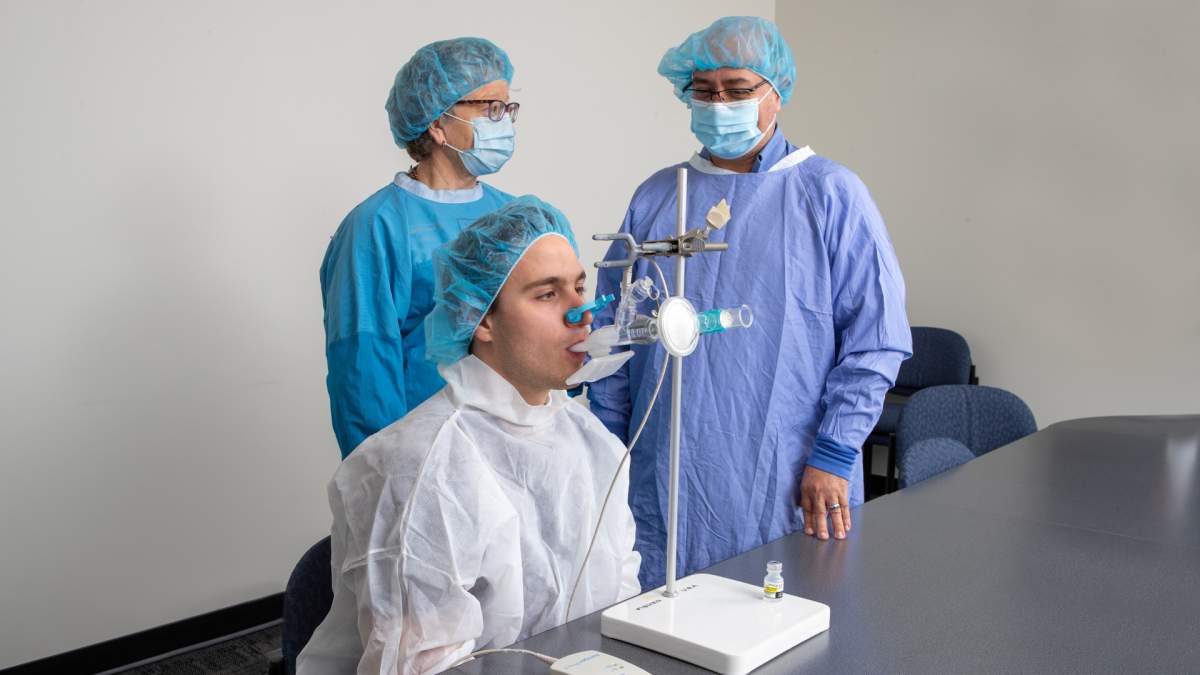
Unlike current vaccines available now, the new generation aerosol will target the lungs and upper airways, where respiratory infections begin.
“We’ve developed a COVID vaccine that contains three of the proteins of COVID-19. The novel part of our vaccine is not only the part around the development of the vaccine cells, but the administration of it,” microbiologist Dr. Fiona Smaill said in a release.
“That’s probably the most exciting part. Instead of giving the vaccine by intramuscular injection, we’re giving that by an aerosol inhalation directly into the lungs using a small jet nebulizer that is very comfortable to administer.”
Phase 1 of the trial, which has the approval of Health Canada, will test the safety and immune potency of two vaccines given to study participants who previously received two doses of a COVID-19 mRNA vaccine, such as BioNTech Pfizer or Moderna.
Matthew Miller, an associate professor at McMaster’s Michael G. DeGroote Institute for Infectious Disease Research, says the new vaccine works similar to the current mRNA vaccines in terms of teaching cells how to make non-harmful components of the virus.
“Essentially what we did was gut the virus of everything it normally uses to make it infectious and we load it instead with the antigens specific to SARS-CoV-2 that we want our immune system to recognize,” Miller said.

Get weekly health news
“That virus is empty. It’s just a shell that’s used to deliver DNA into our cells that teach our cells how to make pieces of SARS-CoV-2.”
A research project at McMaster revealed in June potential applications for a tool in the human immune system that reacts like a “spider web” when engaging microorganisms that can cause SARS-CoV-2, the virus responsible for COVID-19.
At the time, Miller said the potential applications for such a reaction could lead to the manufacture of aerosol and nasal spray technologies that could help head off infections in the body.
Researchers will be comparing two strains of weakened adenovirus — non-enveloped viruses that are vehicles for respiratory infections such as the common cold.
One will be a human adenovirus, the other a chimpanzee adenovirus.
The new vaccines were produced at McMaster’s Robert E. Fitzhenry Vector Laboratory, one of few facilities in Canada with the capacity to develop and produce viral vector vaccines for clinical trials.
“The Fitzhenry Vector lab is a unique resource which made it possible for our team to take innovative research all the way from concept to manufactured vials of vaccine available to boost human volunteers,” says Brian Lichty, an associate professor in the Department of Medicine who is co-leading the vaccine development.
At least 30 healthy volunteers will take part in the study, funded by the Canadian Institutes for Health Research.
Should phase 1 be successful, it’s expected researchers will move on to larger clinical trials, which could potentially lead to broader use.













Comments