Health Canada is warning the public after it seized a number of unauthorized drugs that it said could cause health risks from a Richmond retailer.

The products included a drug labelled as human placenta, and another that was labelled as being derived from stem cells, Health Canada said.
Health officials said products containing human placenta could cause bacterial and viral disease transmission, and that none have been approved for sale in Canada.
The warning noted similar risks with stem cell therapies, and said just one stem cell drug, Prochymal, has been authorized in Canada.
Other seized drugs carried risks of skin irritation or serious damage, kidney damage, hearing loss, toxic buildup and in extreme cases even death.

A full list of potential side effects is available here.
The agency added that the drugs were in packaging labelled in languages such as Japanese and Korean, potentially obscuring key information such as ingredients, dosage and side effects from some customers.

Get weekly health news
The drugs were seized from Before & After Beauty Lab at 115-4231 Hazelbridge Way in Richmond, according to a safety alert posted by Health Canada.
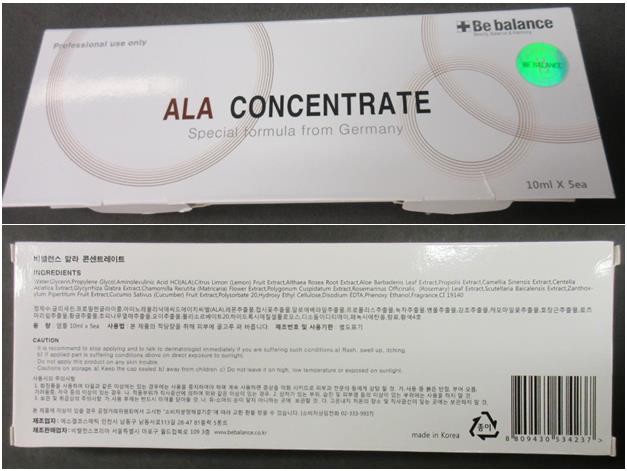
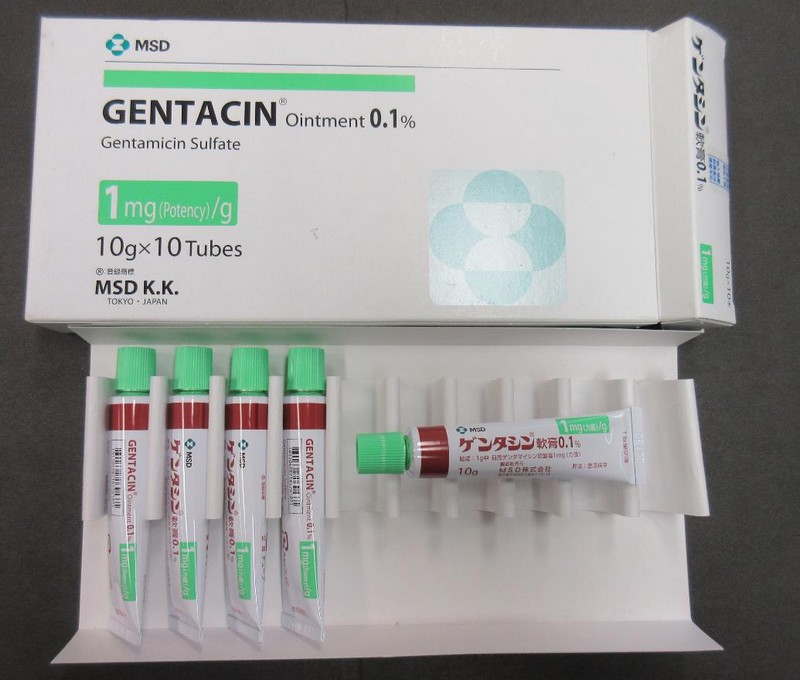
Affected products
- Be balance ALA (aminolevulinic acid) Concentrate
- Dr Maylab “Losheen Stem Cells Therapy” Amniotic Fluid-derived stem cells
- J-Cain lidocaine cream (15.6%)
- Mastelli Placentex (polydeoxyribonucleotide 5.625 mg/3ml injectable solution)
- Melsmon Placenta (Human)
- MSD Gentacin Ointment 0.1% (gentamicin)
Health Canada said anyone who purchased these products from Before & After Beauty Lab or another retailer should not use them.
Anyone who has taken the drugs should consult a healthcare professional, the agency said.
It added that it is working with the Canada Border Services Agency (CBSA) to block any more of the products from entering the country.












Comments