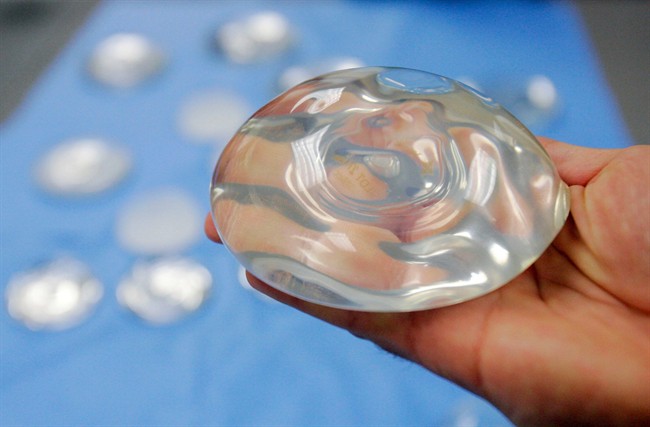
The U.S. Food and Drug Administration (FDA) is linking textured breast implants to a rare blood cancer. The warning comes after nine deaths and more than 350 cases of cancer were reported to American health officials.

Now, the FDA is saying it’s agreeing with the World Health Organization. Last year, the global health body concluded that anaplastic large cell lymphoma (ALCL) – a rare type of non-Hodgkin’s lymphoma – could develop after getting breast implants.
“In 2011, the FDA identified a possible association between breast implants and the development of ALCL … At the time, the FDA knew of so few cases of this disease that it was not possible to determine what factors increased the risk,” the U.S. agency said in a statement.
“All of the information to date suggests that women with breast implants have a very low but increased risk of developing ALCL compared to women who do not have breast implants,” the warning said.
READ MORE: These are the other breast cancer symptoms you should be aware of

Get weekly health news
As of Feb. 1, 2017, the FDA received 359 reports of breast implant-linked ALCL and nine deaths tied to the cancer.
Over the past six years, government health officials looked into the potential link. It’s saying the exact number of cases of cancer tied to breast implants is hard to pinpoint, mostly because there are limitations in how the procedure is reported and there is a lack of implant sales data.
But, for now, the FDA is saying “most data” is pointing to ALCL development with textured breast implants and not smooth ones. This applies to both saline and silicone implants.
It can be treated by simply removing the implant and the capsule surrounding the implant, the warning says. Chemotherapy and radiation are also effective options.
READ MORE: 7 fertility myths and misconceptions Canadian women need to know
So what can women with implants do to keep safe? For starters, pay attention to any symptoms. Patients typically report pain and swelling.
“Follow your doctor’s instructions on how to monitor your breast implants. If you notice any changes, contact your health-care provider promptly to schedule an appointment. Get routine mammography screening and ask for a technologist specifically trained in performing mammograms on patients with breast implants,” the FDA said.
Breast implants don’t increase the risk of breast cancer but they make it trickier to perform and read mammograms, according to the Canadian Society of Plastic Surgeons.
Read the full FDA warning.
carmen.chai@globalnews.ca
Follow @Carmen_Chai





Comments