The Canadian government awarded two of the largest medical supply contracts of the pandemic to importers participating in an invitation-only federal program rather than to Canadian manufacturers offering lower prices, according to a Global News investigation.

The Toronto-area suppliers, BTNX and Switch Health, received contracts adding up to an estimated $2 billion for BTNX and $365 million for Switch Health, when they were participating in the Accelerated Growth Service (AGS), an initiative that helps small Canadian businesses become billion-dollar multinationals. This fast-lane conduit connects participants with officials across all levels of government.
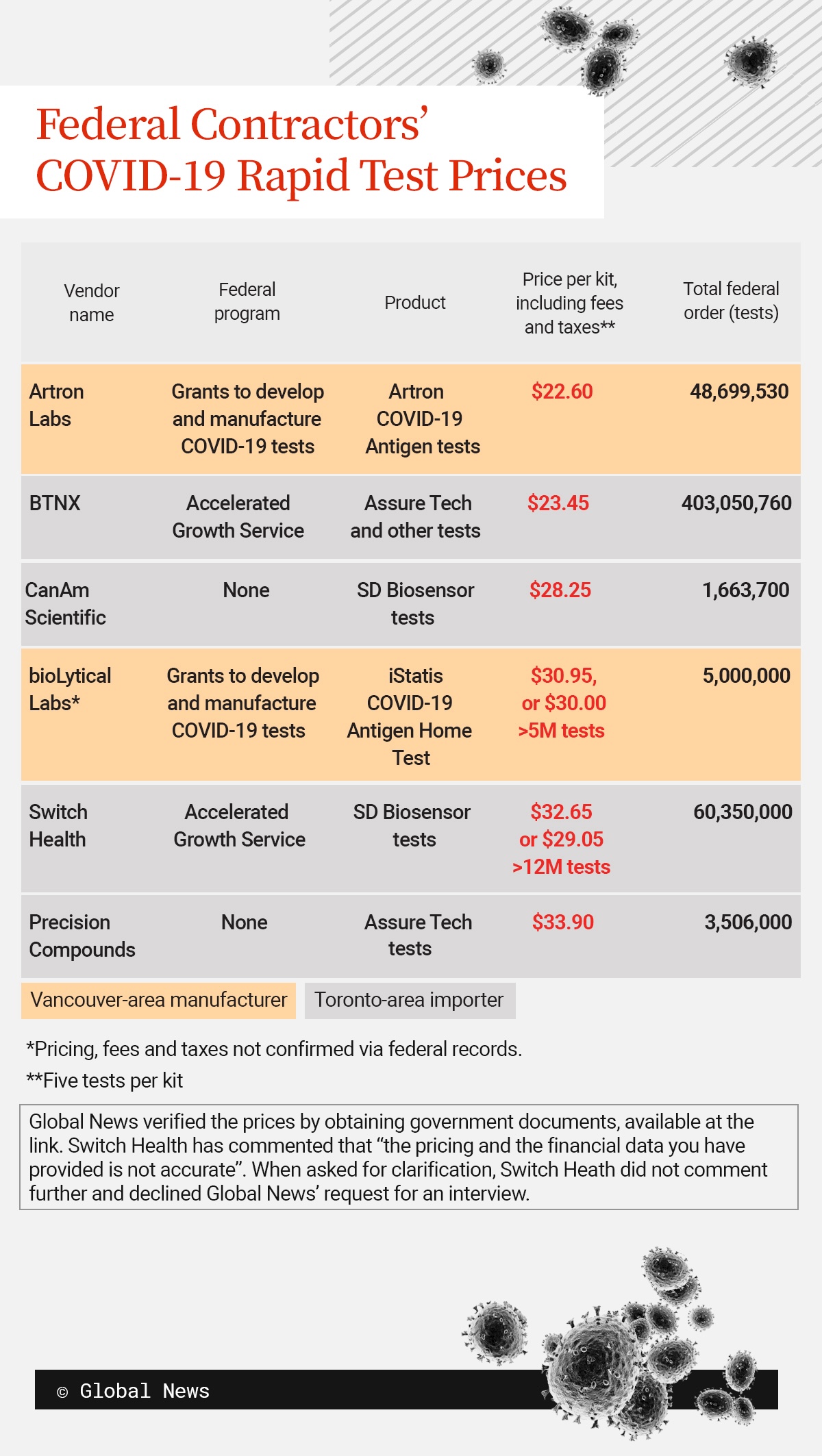
BTNX’s price for a Chinese company’s kit, including fees and taxes, was 85 cents higher than a Canadian manufacturer’s, according to federal documents Global News obtained. The company received orders for 404 million tests, or the largest federal medical supply deal of the pandemic.
Switch Health’s price for a South Korean manufacturer’s kit was $6.45 to $10 higher than the lowest-priced product from a Canadian manufacturer. It received orders for 60 million tests, or the fifth-largest federal medical supply deal.
A spokesperson for Switch Health told Global News, “The pricing and financial data you have provided is not accurate.” The company did not comment further and declined a request for an interview.
Switch Health also won more than $602 million in federal contracts to provide laboratory tests for COVID-19 to international travellers, bringing its gross revenues to nearly $1 billion for 2021.
Ordering the tests from the two importers instead of domestic producers cost taxpayers an additional $56 million or more.
Taxpayers weren’t the only losers in the deal, according to Cenk Ozkan, CEO of the Vancouver-area manufacturer Artron Laboratories.
- Posters promoting ‘Steal From Loblaws Day’ are circulating. How did we get here?
- Canadian food banks are on the brink: ‘This is not a sustainable situation’
- Solar eclipse eye damage: More than 160 cases reported in Ontario, Quebec
- Video shows Ontario police sharing Trudeau’s location with protester, investigation launched
His company and another local operation nearby, bioLytical Laboratories, had each received more than $700,000 in federal funding to develop COVID-19 rapid tests and scale up their manufacturing.
With the market flooded with free tests, Artron and bioLytical had to lay off a total of 630 workers, they told Global News.
Ozkan, whose company was not an AGS member, still wonders why his company did not receive more orders from the federal government.
“Ours was just a better-performing product … a better-priced product, Canadian-made, and we had the capacity that the government paid us to build,” Ozkan said.
Global News has not independently confirmed Ozkan’s claim that Artron’s test performed better than other tests available in Canada, including BTNX’s and Switch Health’s kits.
Global News uncovered the information about BTNX’s and Switch Health’s participation in the AGS during a year-long investigation into medical supply contracts.
In December 2023, Global News reported that BTNX omitted data from an application to Health Canada, boosting an estimate of its test’s ability to detect COVID-19.
BTNX denies that the estimate was inaccurate.
“We have at all times operated with integrity and transparency, and have manufactured and distributed our COVID-19 rapid tests in accordance with Health Canada and international standards,” a spokesperson wrote.
Health Canada stated that it found no reason to question the integrity of BTNX’s application.
On Jan. 7, a team of scientists led by the Public Health Agency of Canada released an initial draft of a new study that, according to the authors, “corroborates manufacturer claims.”
The draft has not yet been reviewed by peer scientists or approved for publication.
The federal government has not publicly disclosed information about its selection process and all companies participating signed confidentiality agreements.
Federal officials have also withheld information on rapid test prices from the public, arguing that suppliers would use the information to jack up rates. Global News obtained it partly through close examinations of redacted freedom-of-information requests and historical records of government websites.
Bulk orders were key to low prices, importers explained to Global News. Large volumes gave buyers leverage to negotiate with manufacturers in China, whose costs were less than $2 per test.
There should be no questions about how or why government contracts were awarded, said Fraser Johnson, a professor of supply chain management at Western University.
Government procurement “has to be a) transparent, b) it has to be fair,” he said.
If AGS participants were favoured over other competitors, that information had to be disclosed to bidders, he said.
BTNX and Switch Health both said that they saw few benefits from their involvement in the program.
“BTNX does not understand Global News’ repeated reference to the AGS program,” a BTNX spokesperson wrote, stating that the company only received “consultative advice.”
Switch Health’s chief strategy officer, Mary Langley, wrote, “The Accelerated Growth Service (AGS) provided no assistance in respect of any federal procurement in which Switch Health was successful.”
Innovation, Science and Economic Development Canada (ISED), the ministry responsible for the program, denied that AGS conferred an advantage to suppliers vying for contracts.
“AGS Innovation Advisors are not involved in the decision-making processes of … other government departments,” a spokesperson wrote.
The ministry’s internal correspondence about how to answer Global News’ questions told a different story.
“Isn’t that what AGS is supposed to do? Help high-performing businesses navigate govt and compete more effectively for grants and contracts?” an ISED employee wrote in notes that Global News obtained via freedom-of-information request.
Welcome to the club
ISED, a sprawling ministry once called the Department of Trade and Industry, deployed the AGS as part of an early pandemic initiative, Canada’s Plan to Mobilize Industry to Fight COVID-19.
The effort tested senior employees’ ability to coordinate their normally siloed teams. Health Ministry officials advised their ISED counterparts, who drew on the resources of the 17 departments within then-ISED minister Navdeep Bains’ portfolio. They deployed the National Research Council, the National Sciences and Engineering Research Council, and seven development agencies to offer grants encouraging Canadian businesses to develop and manufacture masks, gowns, gloves and other supplies.
AGS started inviting rapid test sector businesses to join the program in October 2020.
It was an ambitious move.
With global demand skyrocketing, AGS’s “custom-built” teams of officials could help Canadian businesses grow into industry-leading multinationals and secure a corner of that market.
One of the few barriers to joining is the requirement that an AGS worker or agency nominate a business that is ready for growth, a bureaucratic take on the TV show Shark Tank.
When Global News asked ISED how it ensures that the system does not favour companies with government or political connections, it did not respond.
BTNX was the second rapid test sector business invited to the AGS. The first was LuminUltra Technologies, a New Brunswick laboratory supplies manufacturer.
Under the leadership of CFO Mitch Pittaway, a former lobbyist for the Ford Motor Company, BTNX had been drumming up interest within government in its tests for COVID-19. The small business of about 30 employees was selling repackaged tests purchased from Assure Tech, a manufacturer in Hangzhou, China.
An employee at the National Research Council’s Industrial Research Assistance Program nominated BTNX. Pittaway filled out a form, and 11 days later, BTNX was in.
AGS employees tried to set up BTNX for growth, as its materials promised.
The officials on BTNX’s team roster worked at the Business Development Bank of Canada, regional funding agencies, Export Development Canada, and a half-dozen others, according to records.
AGS also connected BTNX with Ontario’s Ministry of Economic Development, Job Creation and Trade. The ministry contributed to provincial procurement decisions, according to its internal emails.
Next to join the AGS was Switch Health, in January 2021. Due to delayed responses to freedom-of-information requests, Global News has limited information about Switch Health’s participation in the AGS.
An AGS advisor nominated Switch Health, a startup that had launched its services at the pandemic’s outset. It was already providing laboratory testing to at least 35 clients, including Ontario Health, according to the Financial Post.
Scaling up
Beginning in September 2021 and over the following year, BTNX and then Switch Health received contracts adding up to 404 million tests and 60 million tests, respectively.
Canadian manufacturers watched, mystified, as pharmacies handed out importers’ tests, especially BTNX’s lime-green kits, for free.
It was “very strange,” said Ozkan, Artron’s CEO. “We just didn’t expect it because we were in a very good place with the government, (with) meetings every morning.”
Ozkan believed Artron was in the running as federal officials selected rapid test suppliers from among the hundreds that applied to the government’s all-comers request for offers in March 2021. He and executives at competing businesses said no one had told them about the AGS.
Global News’ analysis shows that the nine AGS businesses in the sector were receiving about twice as much funding and were more than twice as likely to receive a federal contract as 17 non-members taking part in other ISED-led initiatives. BTNX and Switch Health were the only enterprises that did not receive any grants. (You can view the grants data online.)
Paul Larson, a researcher in supply chain management at the University of Manitoba, said that when hundreds of millions or billions of taxpayer dollars are in play, he expects that federal workers will complete a background check and visit the applicant’s operations.
Public Services and Procurement Canada (PSPC) confirmed it did neither. Various PSPC spokespeople described other forms of verification its employees had instead relied on.
Larson flagged his concerns about an exchange between Conservative MP Michelle Rempel Garner and Switch Health executives in May 2021, where she repeatedly asked whether Switch Health subcontractors’ employees were incorrectly identifying themselves as nurses. (You can read Global News’ investigation online.)
Procurement workers should closely examine any such allegation, Larson said. If it proved to be true and connected to the company, that could disqualify the candidate from further contracts.
Langley, Switch Health’s spokesperson, wrote, “Switch Health remained compliant within federal contractual requirements, which included compliance with the respective provincial rules. Any statement that claims or implies that Switch Health trained or otherwise encouraged employees to state that they were nurses when the employees did not have appropriate credentials or qualifications is categorically false.”
The buys from both importers went ahead.
BTNX was the first to receive a contract, three months before its competition, in September 2021. The Ontario government also ordered $50 million, and the City of Toronto bought some too.
In total, the company would receive 49.8 per cent of the federal government’s order.
Switch Health won its four federal contracts to supply rapid tests made by the South Korean company SD Biosensor in December 2021, according to records.
Ninety per cent of the nearly $3 billion in contracts the federal government awarded to smaller rapid test suppliers went to importers based in Toronto and Ottawa.
Federal contracts had essentially pushed Artron and other Canadian manufacturers out of the market.
Ozkan and his team were still hopeful officials would place some of the largest orders with their company.
Artron’s test for COVID-19 performed among the best in the world in the German government’s rolling comparison of rapid tests.
BTNX’s representative said the German health ministry’s results should not be depended upon because the researchers used samples that “did not accurately reflect test efficacy.” Switch Health’s tests were not evaluated in the Germany study, though its South Korean manufacturer’s were.
“It should be a point of pride” for Canada, Ozkan said.
Artron could produce 1.4 million tests per day, according to Ozkan and Debby Duan, Artron’s chief operating officer. Its workers prepared a key part of the tests and packaged them.
Anticipating large federal orders, Artron bought the materials to prepare tens of millions more test kits. Ozkan said he did not know at the time that his company had also undercut BTNX on price.
Those materials are still in storage, Ozkan and Duan said. Artron is struggling to make up its losses.
A stamp of approval
ISED did not respond to Global News’ questions about whether the AGS undermined the $40-million effort to boost the rapid test sector.
According to a spokesperson, domestic manufacturing remains an ISED priority.
“The federal government will continue to enhance national capacity to manufacture medical countermeasures … needed for national health security,” he wrote.
Explaining why the federal website indicates that nearly 60 per cent of the rapid tests it purchased were made in Canada, a PSPC spokesperson told Global News that BTNX workers packaged the test kits here.
BTNX told Global News that it imported the kits in their packages. The company’s spokesperson described several steps the company undertook in Canada as it prepared the shipments for distribution to the provinces.
Global News pointed out recently to PSPC and Health Canada that according to PSPC’s definition, the correct total of tests purchased from Canadian producers is less than six per cent of tests.
Health Canada defended the characterization.
“BTNX was selling the product under their own trademark,” its spokesperson wrote. BTNX ordered the kits, so under Canadian law, it is defined as a “manufacturer.”
Barry Hunt, president of the Canadian Association of PPE Manufacturers, scoffed at the explanation.
“The Canadian public is getting duped by government,” he said.
The deal boiled down to officials paying BTNX an extra fee to have its Markham, Ont., address printed on the Chinese manufacturer’s kits, he said.
Hunt called it “Canada washing.”
Hunt, who is CEO of the health care equipment manufacturer Prescientx, and 14 other mask and N-95 respirator manufacturers, are suing the government, claiming that federal officials “enticed” them into joining ISED initiatives and then failed to honour commitments to buy from them.
The rapid test contracts are another example, Hunt said.
A BTNX spokesperson responded that the company “has always been transparent with Health Canada, and has complied with the government’s procurement requirements.”
On behalf of Switch Health, Mary Langley wrote, “Switch Health followed all procurement guidelines and participated in competitive processes for all government-related procurements.”
Don Davies, the NDP health critic and MP for Vancouver Kingsway, called for an independent inquiry into the $10.5 billion in pandemic spending.
The federal government is “resisting accountability and transparency,” he said, though investigations are revealing “sloppiness and incompetence, all the way down to governmental misconduct.”





Comments