Right now, more than 1,600 Canadians have survived their bout with COVID-19, the disease caused by a novel coronavirus.

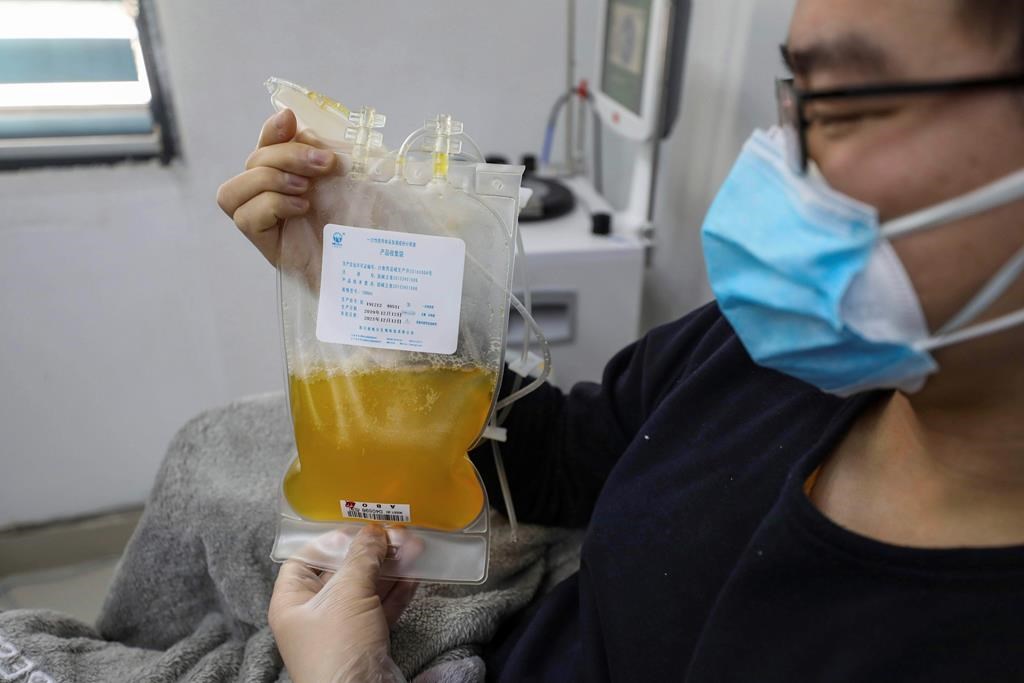
Some doctors think people like these might be able to help others. Specifically, their blood might help.
A group of Canadian researchers is setting up a clinical trial to see whether survivors’ plasma can be harvested and put into other COVID-19 patients to help them beat the disease.

It’s still in the planning stages and likely won’t be recruiting patients for at least a month, but researchers are hopeful that they might be able to find a helpful therapy for the disease — something that doesn’t yet exist.
The idea is simple, even crude, in this age of pharmaceuticals and precision medicine: “This is just blood from one person that’s got protective antibodies into someone else,” said study lead investigator Dr. Donald Arnold.
Antibodies
When a person gets infected with a disease their immune system has never seen before, it takes a few days for their immune system to “get revved up,” said Dana Devine, chief scientist for Canadian Blood Services, one of the organizations involved in the upcoming study.
“What we’ve seen in this coronavirus epidemic is that people can get very high levels of virus in their system before their own immune system has had a chance to make antibodies to attack it,” she said.
Antibodies are proteins that our immune system makes to fight off invaders, she said.
“If you look in my bloodstream right now, I have antibodies against every cold virus I’ve ever been exposed to, every bacterium that ended up in a cut on my skin, I have antibodies against everything I’ve ever been vaccinated against.”

Get weekly health news
The plasma – the clear liquid part of your blood, in which blood cells and other materials are suspended – is a rich source of these antibodies, Devine said.
“The idea behind convalescent plasma is that if you’ve recovered from your COVID-19, your immune system has been revved up and made a bunch of antibodies. And these stay in your plasma at reasonably high levels for quite a few months after you’ve recovered.”
Putting this plasma, with its coronavirus-fighting antibodies, into a sick patient could help them fight off the infection faster, with fewer serious health outcomes like needing a ventilator, or even death, she said.
Or that’s the hope.
Testing the hypothesis
“We just don’t have enough information at this point to say that this is a beneficial therapy,” said Arnold, who is also an associate professor at McMaster University and director of the McMaster Centre for Transfusion Research.
Hence the trial. Researchers are trying to eventually recruit around 1,000 Canadians from across the country to take part in a randomized trial, where some patients will get the blood plasma and some will be treated using the best available care we have now, the same as any seriously ill COVID-19 patient.

This will help to prove whether patients who get plasma do better than those who don’t, Arnold said. The researchers will look at the seriousness of people’s symptoms as well as how many people die receiving the treatment compared to those who didn’t.
There are possible risks to the plasma therapy, too, which could come out during the trial.
“It might even be harmful. There are some theoretical concerns about giving this to patients. It might even make the immune system worse, or they might have reactions,” he said. “So it really needs to be studied rigorously.”
These risks might even include something called antibody-dependent enhancement, where antibodies from an old infection can actually make a person even more vulnerable to subsequent infections, a phenomenon seen sometimes in dengue fever.
But that’s why the study is needed, Arnold said, to figure all this out.
Although the concept of injecting survivors’ blood into other patients has been around since at least the Spanish flu of 1919, and small case studies were done on SARS and MERS patients with promising results, he doesn’t believe that there has ever been a large-scale rigorous trial of this nature.

“The big win, which everyone is after, is that this is an effective therapy and we can get it into the hands of doctors and patients. But we really have to do this rigorously because, as I say, there’s also the possibility that it’s not helpful and maybe even harmful.
“If we don’t do it right, then we’re not doing anyone a service.”
Plasma donors would go through the same kind of screening as any blood donor, Devine said, as well as likely some additional testing to make sure they have a useful amount of antibodies. She doesn’t anticipate any problems finding volunteers – her inbox is already full of curious people, she said.
The research will be done at hospitals and academic hubs across the country, and Arnold said this has all come together at astonishing speed by clinical research standards.
The researchers hope to start in about a month, with results in maybe six to 10 months, he said. While he acknowledges that this might be after the outbreak peaks, he expects there will still be some COVID-19 patients, and any potential therapy could be around in case the disease ever comes back.
The effort and collaboration among researchers to get this going has been remarkable, he said.
“We all see the urgency, we all see the value, and we all see the time-sensitiveness of this. Everyone is so dedicated. I’ve never witnessed anything like this in my life.”


Comments