Several medical devices have been recalled by Health Canada, including one the agency warns could potentially cause death in the most vulnerable patients.

That device is known as the OmniLab Advanced, and it’s typically used by people with sleep apnea — a condition where those affected stop and restart breathing while asleep.
According to the recall notice, the OmniLab Advanced has an alarm to notify users when the device’s ventilator finds an internal error or problem. The agency says products with any lot are recalled or have the model number 1044278.
According to the agency, this includes the device rebooting intermittently, restarting therapy, or even entering an inoperative state with or without reboots.
Health Canada says any of the issues could “result in interruption and/or loss of therapy which may lead to hypoventilation, mild to severe hypoxemia, hypercarbia, respiratory failure/insufficiency, or potentially death in the most vulnerable patients.”
It’s not the only medical device recalled, however.
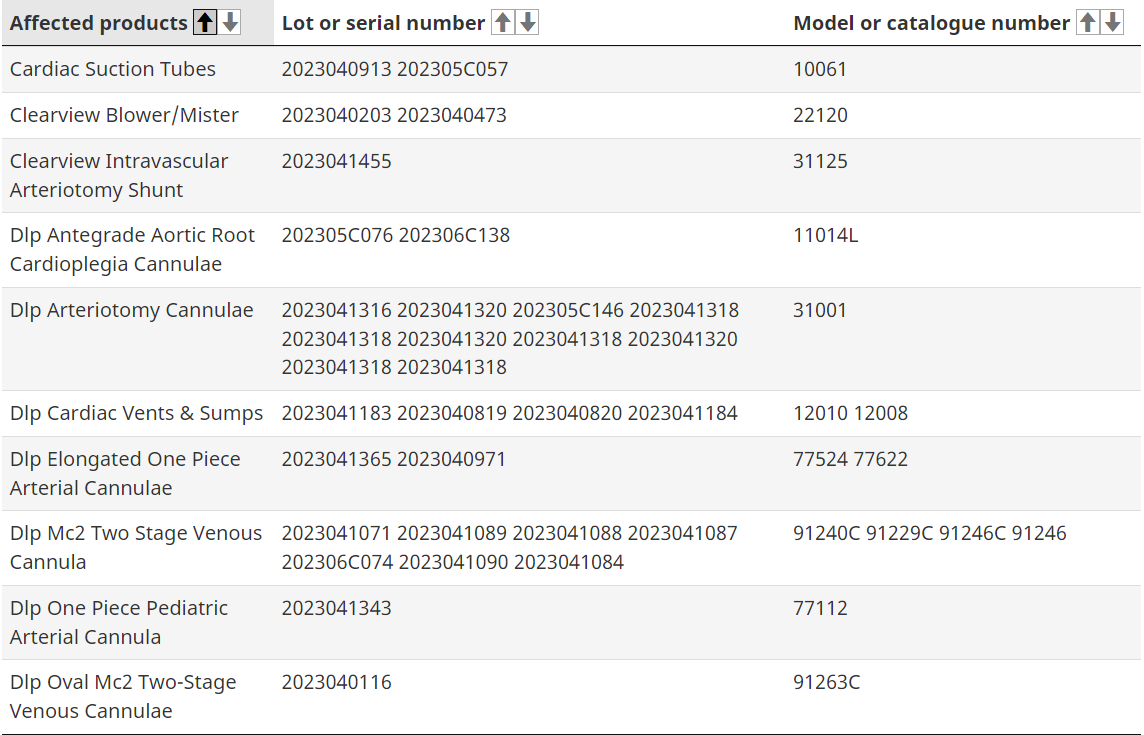
Among the recalls, several of which began last month, are multiple Medtronic cannulae products. A cannula is typically a thin tube inserted into a person’s body cavity like the nose or vein and is often done to drain fluid, administer medication or provide oxygen or even blood.

Get weekly health news
Some of these products include cardiac suction tubes, DLP cardiac vents and sumps, and Arteriotomy Cannulae. Health Canada says specific units are being recalled due to a possible sterility breach.
One lot of the Hugo Ras Surgeon Console, which can provide robotic assistance during surgeries, has been recalled due to 11 incidents of power loss from the main supply. It can lead to permanent loss of being able to teleoperate the system from the surgeon console before or even during surgical procedures.
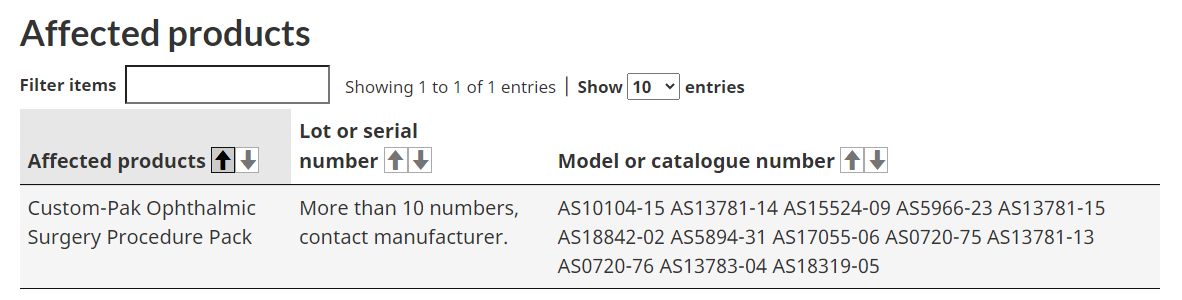
Several models of ophthalmic surgery procedure packs have also been recalled.
The company performed a voluntary medical device field correction following an increased possible risk of inflammation and ocular tissue damage. Health Canada says use of or exposure to the recalled products may cause temporary or medically reversible adverse health consequences.
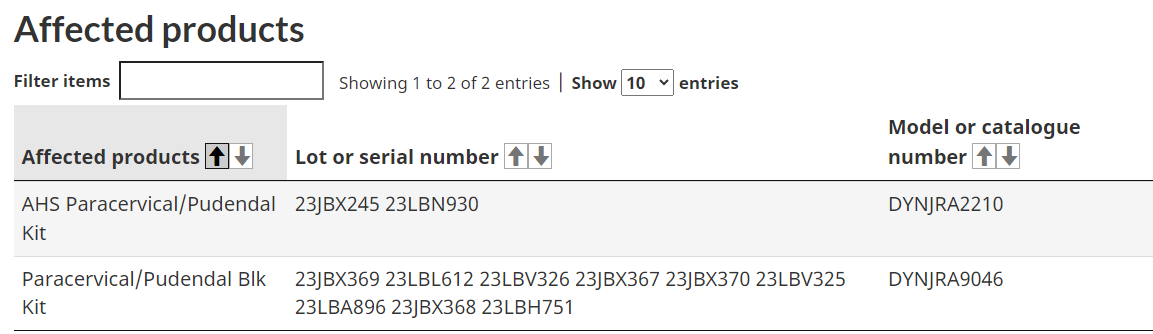
Two models of paracervical/pudendal kits from Medline Industries and Lp, have also been recalled, due to the ring on the trumpet needle guide potentially detaching under excessive pressure.
In each case of product recalls, those using the products are advised to contact the manufacturer if additional information is required.








Comments