TORONTO – A faulty birth control pill, Obamacare’s failed registration website and diluted chemotherapy drugs doled out to hundreds of patients – these were some health care headaches that rocked 2013.

Global News takes a look at five health care blunders that left doctors, patients and health officials scrambling this year.
READ MORE: Year in review: 10 health and nutrition stories of the year
Obamacare’s online rollout: HealthCare.gov, the website where Americans are supposed to be able to sign up for insurance plans, has been marred by delays, glitches, computer issues and registration errors across the board.
Thousands of applicants were allegedly handed over to Medicaid or private insurance because of website glitches and others were denied any coverage. That’s if they were able to make it onto the website in the first place. The site, launched on Oct. 1, went through two months of fixes, but it’s still left U.S. consumers waiting in limbo about their coverage as the new year creeps closer.
Diluted chemotherapy: In March and April, nearly 1,200 cancer patients in New Brunswick and Ontario were warned that they were allegedly given diluted chemotherapy drugs. Two law firms launched a class action lawsuit, claiming that a Hamilton, Ont., company under the name Marchese Health Care improperly mixed chemotherapy drugs cyclophosphamide and gemcitabine.
READ MORE: Almost 1,000 Ontario cancer patients received too-low chemotherapy doses
- Posters promoting ‘Steal From Loblaws Day’ are circulating. How did we get here?
- Video shows Ontario police sharing Trudeau’s location with protester, investigation launched
- Canadian food banks are on the brink: ‘This is not a sustainable situation’
- Solar eclipse eye damage: More than 160 cases reported in Ontario, Quebec
The drugs in question are alleged to have been watered down between three and 20 per cent and administered in diluted states for at least the past year in some hospitals.
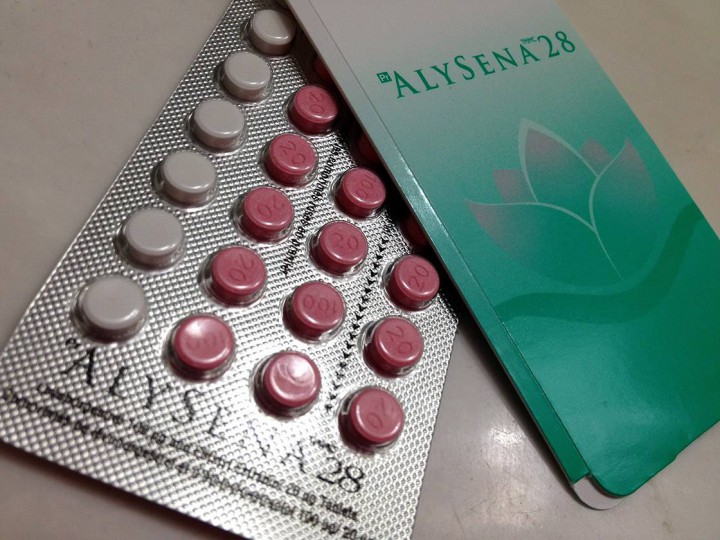
A faulty birth control bill: Following on the heels of the diluted chemo controversy came a scare for Canadian women on the birth control pill Alysena 28. In April, Health Canada warned that the product, distributed by Apotex, had an issue: Alysena is supposed to provide one week of placebo pills in the 28-day cycle. In the recalled packs, there were two weeks’ worth of placebos.
The fallout? An $800-million class action lawsuit was launched, a federal investigation into the timing of the recall, and allegations that about 40 women became pregnant while relying on the faulty product. Apotex, at the time, said it wouldn’t comment on legal proceedings before the court.
Cholesterol calculator controversy: In November, the American Heart Association and the American College of Cardiology put together a new set of guidelines on heart disease. Among them: a new calculator that would determine a patient’s chances of developing cardiovascular disease. The calculator was based on sex, age, race, cholesterol levels, blood pressure, smoking habits or diabetes, among other factors.
Following the new guidelines came a firestorm of contention – critics slammed it for leaving out family history, some called it “risky,” “flawed” and mistakenly suggesting that millions of people need statin drugs when they don’t.
Driving up the cost of drugs: We may not feel the effects on our wallets immediately, but a Canada-European Union trade deal struck in October could force our drug prices to increase in the next few years.
READ MORE: Why critics say the EU trade deal will drive up Canada’s drug prices
One clause brokered between Ottawa and Brussels would provide what’s called “patent term restoration” to big-name drug makers. Critics argue that the move blocks out generic drug companies, handing Big Pharma an extended monopoly that’ll leave consumers without a cheaper option.
They estimate that this two-year extension could result in Canadians losing millions of dollars a year. A generic drug usually costs only a quarter of its brand-name counterpart.
An honourable mention: A federally funded plan in the U.S. to feed students healthier lunches was canned in 524 schools this year. School nutrition officials said the new standards for healthier food were difficult and expensive to follow, critics said the government shouldn’t be dictating how kids eat and kids — as predicted — said that clean eating just wasn’t as tasty.
READ MORE: 500 U.S. schools drop out federal lunch program due to healthier requirements: USDA
With files from David Shum, Global Toronto, the Canadian Press and the Associated Press
carmen.chai@globalnews.ca
Follow @Carmen_Chai




Comments