Canada is preparing a “dry run” for the delivery of the Moderna coronavirus vaccine officials say will be key for reaching remote Indigenous communities.

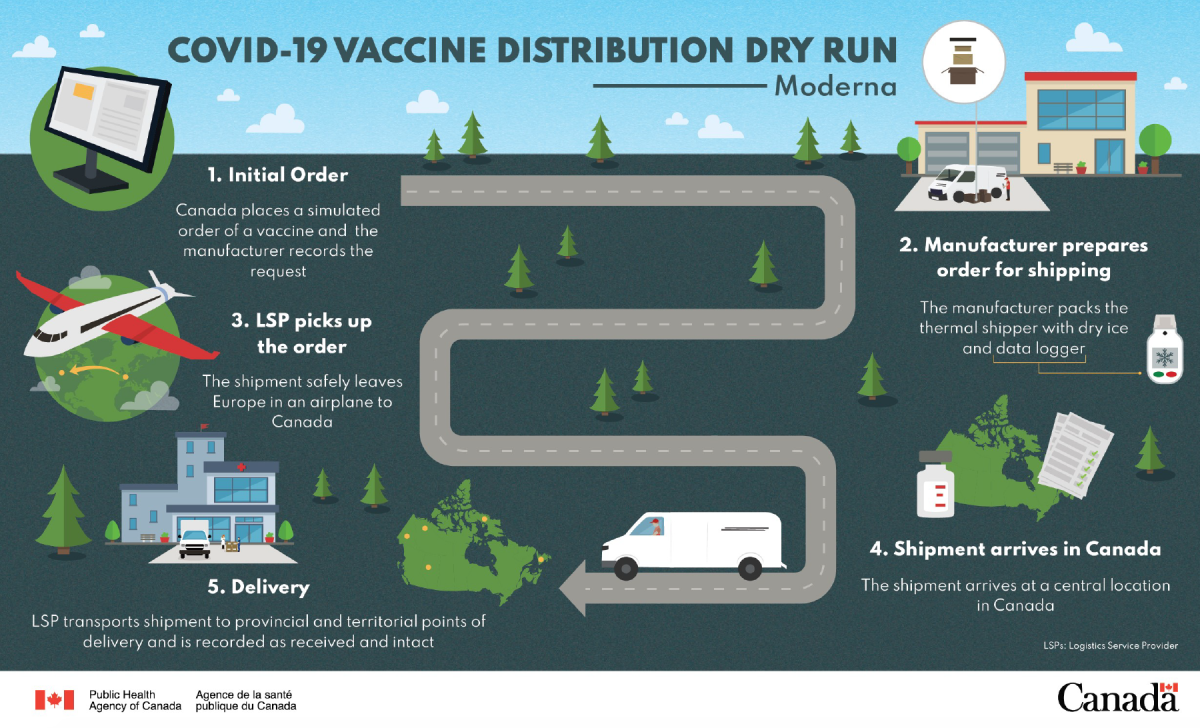
Speaking to reporters on Wednesday, Major General Dany Fortin said the dry run, or rollout practice, was similar to the one performed last week by the Canadian government for the Pfizer and BioNtech vaccine, which involved confirming vaccine orders, and testing shipping, tracking, delivery and storage.
Fortin added this will make it easier to deliver Moderna’s vaccines to locations identified by the provinces and territories so they can be rolled out quickly, pending approval from Health Canada.
“We’re taking deliberate steps to ensure the safe and efficient distribution of the Moderna vaccine candidate across the country,” he said.
Moderna has agreed to supply Canada with up to 56 million doses of its vaccine, to be delivered next year.
Of those, 168,000 shots could arrive by the end of December, if they are green-lit by Health Canada.
Prime Minister Justin Trudeau said Tuesday they could be delivered 48 hours after they’ve been authorized.
Moderna’s vaccine can survive in normal freezers, making it easier to ship to remote locations than Pfizer’s vaccine, which needs to be refrigerated at -70 C until its injection.

Get weekly health news
For the territories, the Moderna vaccine will be the first available to them, after the decision was made to forego sending Pfizer’s vaccines due to refrigeration concerns.

In preparation for the vaccines, Fortin said -20 C freezers were delivered to the territories to ensure the vaccines could be properly stored, along with syringes and other equipment needed for vaccine rollout.
He added that provinces and territories were “moving to scale up to around 70 points of use” across the country in coming weeks.
The freezers will be able to store the Moderna vaccine, which is better suited to remote locations than the Pfizer vaccine, and Fortin said “thousands” more people are being trained to prepare and administer the vaccines.
- Porter flight from Edmonton loses traction, slides off taxiway at Hamilton airport
- Coffee-hockey combo — or breakfast beers? — for bleary-eyed Olympic fans
- Canada warns First Nations people to carry passport when crossing U.S. border
- Canada’s incoming top doctor says restoring public trust a top priority
“We’ll deliver the Moderna vaccine to the location specified by provinces and territories so that they can commence immunization as quickly as possible once it’s approved and available,” he said.
To date, health officials have confirmed 6,390 COVID-19 cases on First Nations reserves, according to numbers provided by Indigenous Services Canada.
The government agency said the percentage of Indigenous peoples living on reserves were testing positive for the virus at a rate of one-half of Canadian’s general population as of Nov. 9.
Dr. Evan Adams, deputy chief medical officer with Indigenous Services Canada, said COVID-19 cases had risen to “alarmingly high numbers” in Indigenous communities in Alberta, Saskatchewan and Manitoba.
“It is imperative that First Nations, Inuit and Metis be partners with provinces and territories in the co-planning for culturally safe and equitable vaccine access in both rural and urban areas,” he said.
“Vaccines will be delivered to northern and Indigenous communities once it has received regulatory approval from Health Canada and a decision is expected soon.”









Comments
Want to discuss? Please read our Commenting Policy first.