The Food and Drug Administration (FDA) recently approved a drug for smallpox — a disease that was eradicated nearly 40 years ago.

On July 13, the agency announced it approved Tpoxx, the first drug that specifically treats smallpox. Smallpox was eradicated from the world in the 1980s thanks to vaccine efforts.
Despite this, there are concerns that the virus that causes smallpox could be used as a bioweapon, the FDA said.
“To address the risk of bioterrorism, Congress has taken steps to enable the development and approval of countermeasures to thwart pathogens that could be employed as weapons,” Dr. Scott Gottlieb, the FDA commissioner, said in a statement.
Smallpox has not technically been wiped off the planet as some stocks of the virus still exist in labs in the United States and in Russia.

Get weekly health news
But some governments believe there is a risk that the virus exists in other places other than these laboratories and could be deliberately released to cause harm, according to the according to the World Health Organization (WHO).
WATCH: Edmonton research team raises troubling questions over resurrecting horse pox

For example, in 2014 a government scientist cleaning out an old storage room at a research centre near Washington found decades-old vials of smallpox packed away and forgotten in a cardboard box.
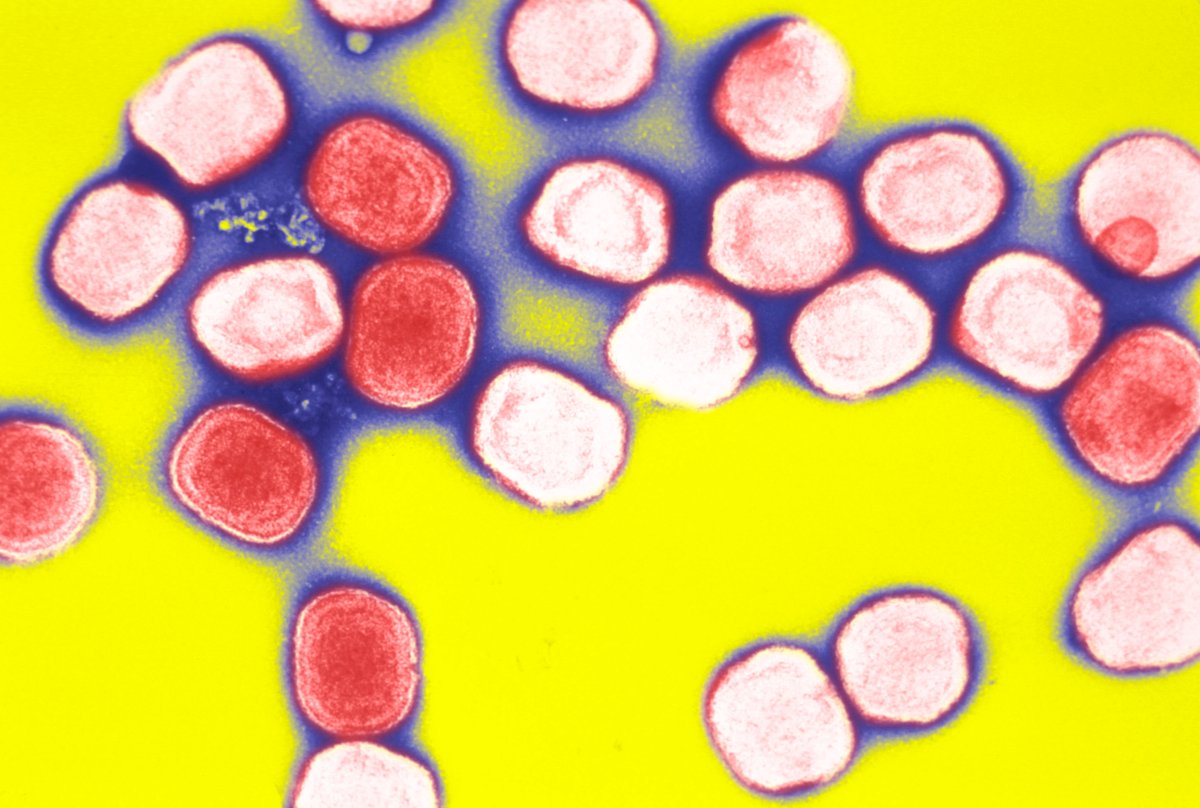
Because routine smallpox vaccination stopped after 1980, very few people are vaccinated against the disease, which is very contagious and kills almost a third of people who get it, WHO stated.
And unlike measles and whooping cough, vaccines for smallpox are too dangerous to give the general public, Dr. Peter J. Hotez, former president of the Sabin Vaccine Institute told the New York Times.
Smallpox killed about 300 million people worldwide in the 20th century before its eradication. Symptoms include fever, fatigue and pus-filled sores.
First approved treatment of virus
Tpoxxis is the first approved treatment specifically for the virus.
The new drug for smallpox was tested in animals infected with viruses that are closely related to the smallpox virus, the FDA said. It was not tested on people infected with similar viruses.
“More animals treated with TPOXX lived compared to the animals treated with placebo,” the FDA said in the statement.
Tpoxx was approved under the FDA’s Animal Rule, which allows animal studies to be used to support approval when it is not feasible or ethical to conduct studies of the drug’s effectiveness in people. However, the drug was tested for safety in more than 350 healthy people who did not have smallpox.








Comments