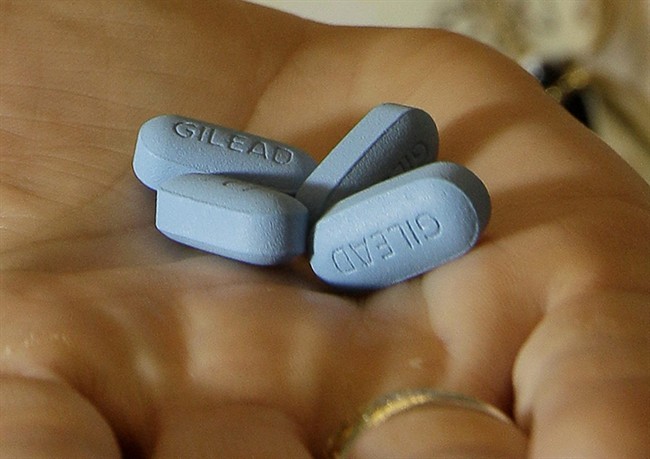
Health Canada has quietly approved an HIV drug as a preventative therapy to keep the virus at bay for people at high risk. Studies suggest that Truvada can cut the risk of HIV infection by 90 per cent, when taken before or after sex.

Gilead Sciences Canada, the makers of Truvada, say the federal agency approved the drug as a pre-exposure prophylaxis (PrEP). Doctors who run HIV/AIDS prevention clinics say the move is a leap forward in making therapy options more accessible.
“We should do everything in our power to enable the prevention of HIV, and that’s just the humane thing to do. It’s also the effective thing to do,” Dr. Isaac Bogoch, an infectious disease expert who runs one of Canada’s biggest HIV prevention clinics out of Toronto General Hospital, told Global News.
“I can’t tell you how happy I am. Now, we feel we have the blessing of Health Canada to do what we’ve been doing all along,” he said.
READ MORE: Pills before and after sex can help prevent HIV, study finds
Truvada is taken orally once per day. It contains two medications and it’s used as part of a drug cocktail regimen to treat HIV-positive cases. In this case, it’s being backed by Health Canada as a preventative measure.
If some patients are identified as “high risk,” they could use Truvada to stave off the virus. Those considered at high risk may have a partner who is HIV positive, or they could be engaging in “risky” behaviour such as unprotected sex with partners who may be exposed to HIV or sharing injection needles, Bogoch said.
- Solar eclipse eye damage: More than 160 cases reported in Ontario, Quebec
- 3 women diagnosed with HIV after ‘vampire facials’ at unlicensed U.S. spa
- ‘Super lice’ are becoming more resistant to chemical shampoos. What to use instead
- Canadian man dies during Texas Ironman event. His widow wants answers as to why
It’s already been approved for PrEP in the United States as of 2012, as well as in Kenya and South Africa in 2015. It’s in the works in Australia, Brazil, Peru and Thailand, too.
READ MORE: HIV prevention drug approved in the US but not yet in Canada
Gilead Sciences told Global News last year that it applied for Truvada to be used as a prevention method in August 2015.
It was the first drug to be approved by the U.S. Food and Drug Administration to reduce the risk of HIV infection in high-risk populations. It’s also the first to receive the nod by Canadian officials.
“Multiple clinical trials have demonstrated that Truvada for PrEP is effective at reducing the risk of HIV infection acquired through sexual exposure,” Dr. Cecile Tremblay, of the University of Montreal’s department of microbiology, said in a statement.
“The number of new HIV infections in Canada has remained steady over the past several years and it is exciting to consider the potential impact of a new tool to help lower the rate of HIV infections in the future,” Tremplay explained.
READ MORE: Why the United Nations is adopting Canadian scientist’s HIV strategy
Right now, patients pay out of pocket or through private insurance for Truvada therapy, according to Dr. Jason Brunetta, who runs a prevention lab out of Maple Leaf Medical Clinic.
The drug comes with a hefty price tag – about $12,000 to $15,000 annually.
A Health Canada approval ushers in potential for provinces to provide funding to cover the drug.
“The official approval may improve access for patients. It may expand the reach and the availability of the drug,” Brunetta said.
READ MORE: A made-in-Canada health strategy is making waves worldwide – just not in Canada
There were about 2,570 new HIV infections reported in Canada in 2014, according to the Public Health Agency of Canada.
Canadian studies have suggested that Truvada taken daily as a precautionary measure could reduce the risk of HIV infection anywhere from 90 to 100 per cent.
“We know that if people are able to adhere with it pretty well, take their pill on most days, that it can achieve extremely high levels of protection, north of 95 per cent reduction in risk. One study even estimated something closer to 100 per cent reduction in risk,” Dr. Darrell Tan, an infectious disease physician, from St. Michael’s Hospital told Global News.
READ MORE: HIV isn’t easily transmitted by sex, Canadian doctors say
Past studies have looked at those who take a daily pill and what impact that daily dose has on prevention. Tan’s study, released last December, looked at what happened in high-risk populations that took the medication on an “on-demand” basis.
Participants took two pills hours or days before sex and two pills after, one at 24 hours and the next at 48.
The results suggested that those who took Truvada “on demand” were 86 per cent less likely to get infected.
carmen.chai@globalnews.ca
Follow @Carmen_Chai



Comments