McMaster University researchers say the development of a “spit test” that can be used to identify potential COVID-19 infections in 10 minutes is very close to a worldwide release.

The Hamilton-based scientists, who’ve been working on the project for close to a year, say government funding and backing from a health-care technology development company have paved the way for the new saliva-based antigen test to hit the marketplace within the next year.
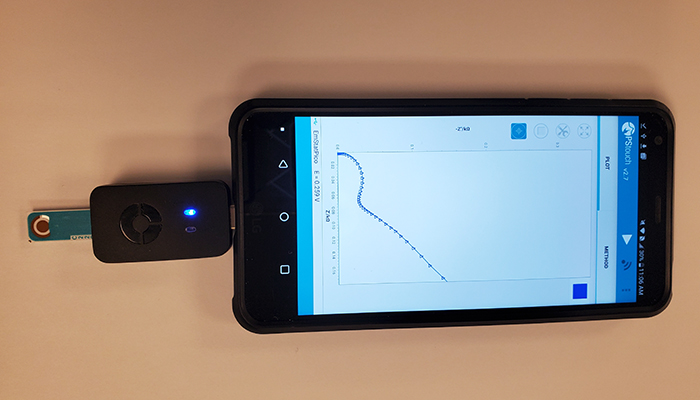
The key component in the so-called “aptamer-based” test is a smartphone which allows for accurate readings within minutes and potentially eliminates the need for time-consuming laboratory work.
“So you have a reader, a handheld reader and a cartridge that plugs into it,” said Leyla Soleymani, associate professor in the department of engineering physics at McMaster, on 900 CHML’s Good Morning Hamilton.
“It can diagnose rapidly a number of infectious diseases with the focus right now on COVID 19. But it can be extended to other infectious diseases.”
Soleymani said the collection is similar to the current free rapid antigen tests Ontario has been distributing and involves depositing a drop on a cartridge that plugs into an electronic reader.
“So it’s no longer you visually see a line, but it can go through an app where it can tell you clearly whether you have the disease or not,” Soleymani said.
“The nice thing is that you can buy a cartridge for COVID 19, but then you can buy a cartridge for another infectious disease, say influenza.”
The researchers are targeting the test for doctor’s offices or hospital clinics with the idea of diagnosis and prescription through a single patient visit.
Norovirus, C. difficile, and a number of other respiratory virus infections are the range of non-COVID test kits they hope to have as expansion options in the near future.
“We want to be able to streamline the process of adapting the technology to other infections so we can react quickly to new threats, whether they are variants of COVID or other forms of infection,” McMaster biochemist Yingfu Li said.
“By anticipating the future need for testing, we will be able to react much more quickly when specific new threats emerge.”
Researchers say Zentek Ltd., specializing in nanotechnology and healthcare, is the licensee of the new test and is expected to invest more than $1 million in the next five years to scale up production of the test components for manufacture at a commercial level.
The Natural Sciences and Engineering Research Council of Canada is providing $1.5 million in funding through a pair of grants.
Soleymani said the timeline for the public to see a first use of the product is within a year or so, targeting the hospitality sector and long-term care workplaces, and with an eventual release for consumer use at home.





Comments