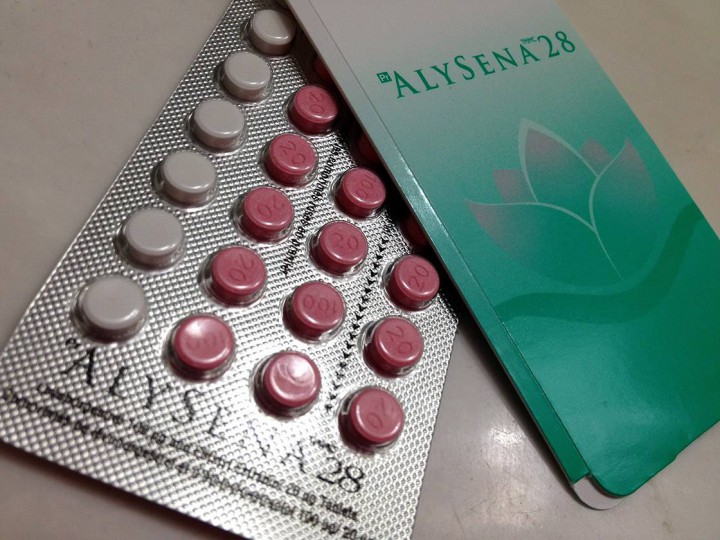
TORONTO – If you had a prescription filled for the birth control pill Alysena 28 you should check the lot number following an urgent recall posted this week.

Health Canada is warning women that the product distributed by Apotex has an issue with the placebos in packages from Lot number LF 01899A.
Read the Health Canada recall posted on April 8.
According to Apotex, Alysena is supposed to provide one week of placebo pills in the 28-day cycle. In these packages, the lot number contains two weeks’ worth instead. The first two rows are placebos, the urgent recall note says.
The recall warns that ingestion of only 14 tablets instead of 21 “would most likely result in reduced efficacy for contraception.”
It adds that the “possibility of unplanned pregnancy cannot be ruled out.”
Read Apotex’s urgent recall notice here.
Placebos are used in birth control products so that patients remember to take the pill consistently.
London Drugs informed patients of recall over the weekend
- A ‘zombie’ virus is raging among raccoons. What to know
- Ultra-processed food tied to higher risk of early death, study finds. What to avoid
- N.S. couple felt they won ‘doctor lottery’ after years on wait-list. Now they’re back on it
- Panera to remove ‘Charged Sips’ drink from Canada amid wrongful death lawsuits
On Friday afternoon, when John Tse saw the recall notice, he says he knew he had the duty to inform his patients that they may be taking pills that weren’t helping them keep pregnancy at bay.
“When I sat back and looked at it, I thought it was prudent to contact the women in our database,” he told Global News.
Tse is Vice President of Pharmacy for West Coast company London Drugs. He spent the weekend working around the clock with information technology workers and about a dozen more pharmacists scouring their databases to find each patient who could be taking the recalled batch.
They were able to determine when shipments of the drug arrived and isolated a group of 350 women that could have been handed the product.
They spent the next two days phoning each of the patients on their lists, explaining what had happened. They offered patients free pregnancy tests, Plan B pills and help to put patients in touch with doctors if they requested it.
Some patients were 18 and younger. In those cases, the pharmacists contacted their doctors instead to maintain privacy.
London Drugs blogged about the incident by Saturday.
“Although this recall is currently a Type 2 recall and does not require pharmacy to contact patients, due to the potential seriousness of the recall, and in an abundance of caution, care and concern for our customers, London Drugs has chosen to be proactive in advising our customers and patients of the recall,” the blog post read, linking off to the urgent notice.
Class 1 situations require “reasonable probability” that the use of the drug will cause serious health consequences or death. Class 2 could lead to “temporary adverse health consequences,” Health Canada explains on its website.
By Monday morning, Tse said the Health Canada notice included types 1 and 2 in its hazard classification of the situation.
“We were not challenging anybody on it being a type 1 or type 2 classification. We felt that we had to champion this for the patients because the patients needed to know,” Tse told Global News.
Recall spans from coast to coast
The Canadian Pharmacists Association says that ethically, pharmacists would tell their patients if a drug was recalled and could affect their well-being.
“This is a really serious recall event because of the potential implications it has on patients,” Phil Emberley, director of pharmacy innovation with the CPA, told Global News.
The pharmacist with more than 25 years of experience says situations like this occur very infrequently.
Health Canada says wholesalers, distributors and retailers of the pill are in British Columbia, New Brunswick, Newfoundland, Nova Scotia, Ontario, Prince Edward Island and Quebec.
Apotex told Global News that it would be releasing a statement Monday. It did not respond to any questions.
– With files from Paula Baker
carmen.chai@globalnews.ca
Follow @Carmen_Chai


Comments