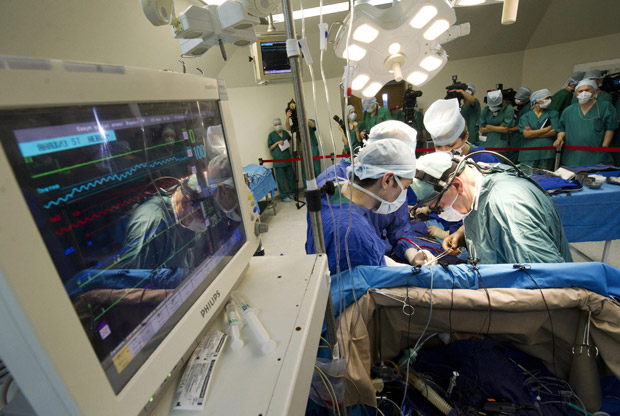
The Nova Scotia Health Authority is warning patients of a low risk of infection associated with heart surgeries over the past four years.

READ MORE: Open-heart surgery patients at Montreal Heart Institute could suffer infections
Devices used to heat and cool blood during heart surgeries within the NSHA and the IWK Health Centre have been linked to a rare bacterial infection in other parts of the country caused by Mycobacterium chimaera, also known as non-tuberculous mycobacteria.
No cases have been reported in Nova Scotia, but those exposed to the devices will be receiving letters for further information.
A release by the NSHA said there was a “slight possibility” contaminated water in the devices was transferred “through the air” in operating rooms at both the IWK and the QEII Health Sciences Centre.
The Health Authority said chances of becoming infected are less than one per cent, but an advisory released Monday is to inform patients of the new low risk.
Anyone with concerns should speak with their family doctor.
READ MORE: Open-heart surgery patients at 6 Quebec hospitals at risk of infection
People who contract the infection, commonly found in nature, rarely makes healthy people sick, according to the Centre for Disease Control and Prevention. But Health Canada warns while “typically not harmful,” in rare cases it can cause potentially life-threatening infections in very ill patients.
Last month, six hospitals in Quebec started contacting patients after two patients who underwent open heart surgeries at the Montreal Health Institute were diagnosed with the infection.
There are no plans by the NSHA to replace the devices, which are used in hospitals across North America and Europe. The release said both organizations are following all recommendations “made by both the manufacturer of these devices and Health Canada.”
- Toronto Pearson gold heist: Ontario man arrested at airport after arriving from India
- Capital gains changes could have ‘irreversible’ effects, business groups warn
- Renters so far more ‘vulnerable’ than homeowners amid higher interest rates. Why?
- After tornado outbreaks in the U.S., could Canada see similar storms?


Comments