The Ontario government is expanding access to a treatment option for cystic fibrosis patients.

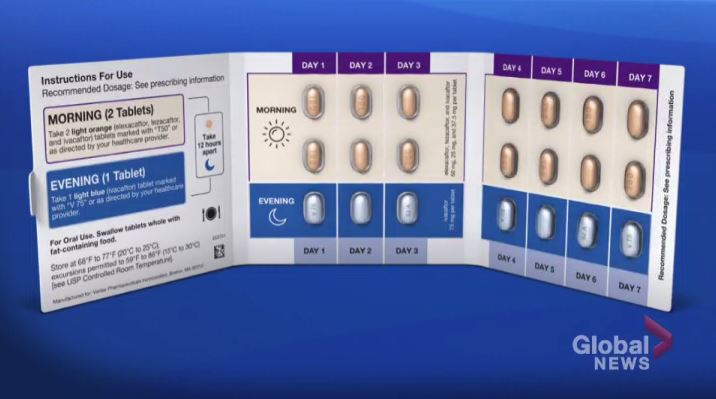
The Ministry of Health said it is expanding coverage for Trikafta — a treatment for cystic fibrosis — to include all residents aged six and over through a publicly-funded drug program.
According to the government, cystic fibrosis is a “rare and progressive genetic disease” that causes thick mucus to build up in the lungs, digestive tract and other areas of the body.
The disease causes persistent lung infections and loss of lung function.
“The province is also changing the eligibility criteria to remove the measurement of patients’ lung function in order to further reduce barriers for cystic fibrosis patients to access lifechanging treatments,” a news release read.
Previously, the Ministry of Health said the treatment was only available to those 12 and older.

Get weekly health news
“Following new recommendations by the Canadian Agency for Drugs and Technology in Health (CADTH), Ontario is now the first province to expand access to youth aged six to 11,” the release read.
According to the government, one in every 3,600 children born in Canada has cystic fibrosis. Approximately 1,500 Ontarians have the disease.
The ministry said eligible patients and their families should reach out to their health-care providers to determine if Trikafta is “the right treatment option” for them.
Ontario’s Minister of Health Sylvia Jones said expanding coverage will “ensure that more children with cystic fibrosis will be able to benefit from this innovative, lifechanging treatment sooner.”
“Providing patients with more options for treatment is another step in our government’s work to build a stronger health care system for all Ontarians,” Jones said in a statement.
Kelly Grover, president and CEO of Cystic Fibrosis Canada said Friday’s news will “change the trajectory of the disease and the future for many children and adults in Ontario who live with cystic fibrosis.”
“Ontario was one of the first provinces to fund the drug for those 12 years of age and older last year, and today has continued to recognize Trikafta’s extraordinary, transformative value, by expanding coverage of Trikafta to include children ages six to 11 years old,” Grover said.
“We are pleased to see that the restrictive start criterion has also been removed, enabling more people to access the drug. We celebrate this news alongside our CF community in Ontario, who have worked tirelessly for this day.”
According to the ministry of health, at list price Trikafta costs around $300,000 each year per patient.







Comments