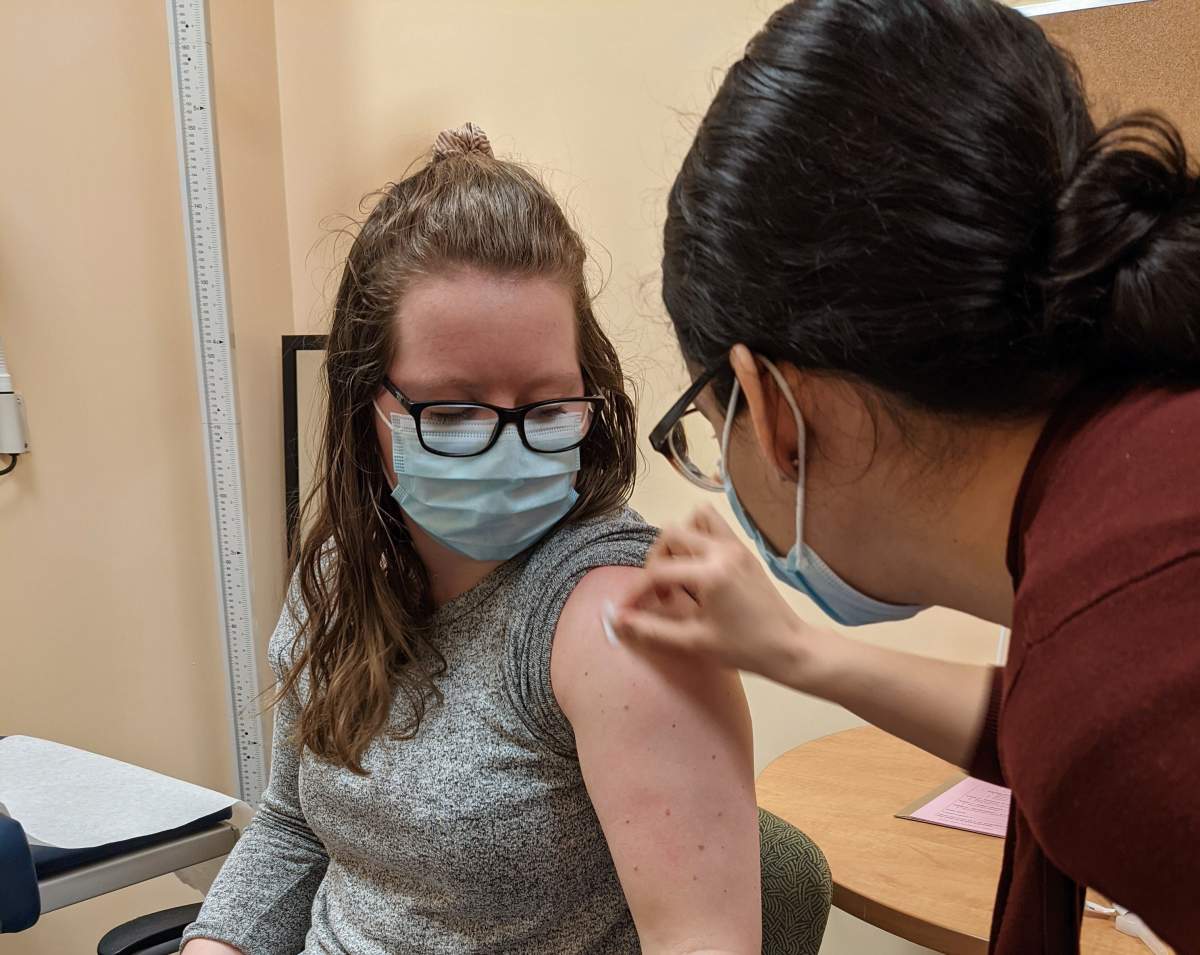
A made-in Alberta COVID-19 vaccine marked a major milestone Thursday with the first recruits involved in a clinical trial injected with the vaccine.

Edmonton-based Entos Pharmaceuticals, led by University of Alberta researcher Dr. John Lewis, began Phase 1 of its clinical trial Thursday.
The first participants were injected with the Covigenix VAX-001 vaccine at the Canadian Center for Vaccinology in Halifax, N.S.
The centre will enrol up to 73 health adults aged 18 to 55 and 65 to 84. Entos Pharmaceuticals anticipates within two months it will have early indications of the vaccine’s safety profile.
It’s hoped the DNA-based vaccine will eventually be approved for use by Health Canada.

Get weekly health news
“We need to get 16 billion doses worldwide to beat this pandemic, and we believe DNA is the perfect way to approach that,” Lewis said in a news release Thursday.

Earlier this year, Lewis said the Entos vaccine has shown a “strong neutralizing antibody response” in pre-clinical testing and is expected to provide protection against the original strain of SARS-CoV-2, as well as its main variants currently circulating worldwide.
Researchers also believe the vaccine will provide protection after a single dose.



Comments
Want to discuss? Please read our Commenting Policy first.