TORONTO – A birth-control pill recalled because of a packaging mistake was sold across the country, says the company that distributes the oral contraceptive, potentially affecting tens of thousands of Canadian women.

On Tuesday, Health Canada issued a notice of recall for Freya-28 after a pharmacy reported that a package had been found that contained a placebo pill in place of an active one.
Scroll down to see the original press release
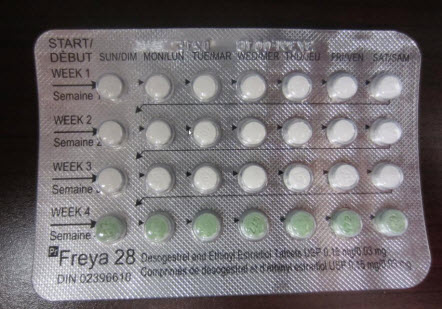
Packages of the pills sold by Mylan Pharmaceuticals should have 21 white pills containing active ingredients, laid out in three rows, and a single row of seven green placebo pills.
Health Canada says missing one or more active pills could reduce the contraceptive’s effectiveness and result in an unplanned pregnancy.
A spokeswoman for Mylan Pharmaceuticals said two lots of Freya-28 – 3739F001B and 3739F002B – have been recalled. The company’s other birth control product, Freya-21, is not affected.
The lots contained a total of more than 79,500 individual blister packs of the 28-day contraceptive, said Nina Devlin. Almost 76,300 of those had been distributed across Canada between May 10 and Aug. 22, when the packaging mix-up was reported to the company.

Get weekly health news
The drug is packaged for Mylan by Famy Care Ltd., a Mumbai-based pharmaceutical company.
Consumers using Freya-28 can return unopened packages to their pharmacists and they are being asked to report any adverse reactions potentially related to recalled pills to Health Canada.
The latest incident comes after a major birth-control drug recall involving Alysena 28 earlier this year.
One lot of the product contained too little active drug and too much placebo, leaving women who took it vulnerable to becoming pregnant.
The lot sold by generic drug maker Apotex contained about 50,000 faulty packets, which were sold across Canada.
Women using birth control pills take the drugs for 21 days of each menstrual cycle. Because of the risk that they might not remember to resume taking their pills at the right time, many oral contraceptives are packaged with a pill for each of the 28 days in a cycle – 21 are drug and seven are placebo.









Comments
Want to discuss? Please read our Commenting Policy first.