MannKind Corp. said Wednesday that its inhaled insulin Afrezza met the main goals behind two late-stage studies, as the drug developer prepares for another push to get regulatory approval for its diabetes treatment.

Shares of the Valencia, Calif., company climbed $1.20, or 18 per cent, to $8.06 in morning trading, striking a 52-week high of $8.70 just after the open.
MannKind said preliminary results from the research showed that type 2 diabetes patients taking Afrezza and the widely used generic drug metformin saw better reductions in long-term blood sugar levels, called A1c levels, than those taking an oral medication. The research also showed that Afrezza helped produce reductions in blood sugar levels comparable to another insulin treatment in patients with type 1 diabetes.
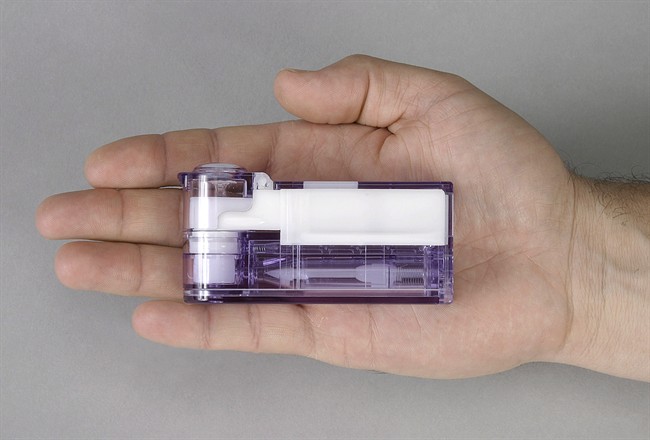
Afrezza is a fast-acting insulin powder that comes in single-use cartridges delivered through an inhaler named Dreamboat.
MannKind, which has no drugs on the market and lost $46.1 million in its most recent quarter, first filed for Food and Drug Administration approval of Afrezza in March 2009. The FDA then told the company in early 2011 to run more clinical studies. The agency wanted MannKind to use the studies to compare its new inhaler with one called MedTone that it used in previous studies.

Get weekly health news
MannKind said Wednesday that the changes in lung function that it saw in the group using the Dreamboat inhaler were no different than those seen in patients using the MedTone device.
The company plans to use results from these studies in another application for approval it expects to submit to the FDA early in the fourth quarter.
Several other companies have failed to make inhaled insulin work commercially. In 2007, Pfizer Inc. discontinued its inhaled insulin Exubera after it failed to gain ground on the market. In 2008, Eli Lilly and Co. ended its development program, citing regulatory uncertainty.
Diabetes is a chronic condition in which the body either does not make enough insulin to break down the sugar in foods or uses insulin inefficiently. It can cause early death or serious complications like blindness, a stroke, kidney disease or heart disease when blood sugar climbs too high and damages organs and blood vessels.
Type 1 diabetes is usually diagnosed in children and young adults. In those cases, the body does not produce insulin. In type 2 diabetes, the most common form of the disease, the body does not use insulin properly.
Demand for drugs that treat diabetes is climbing as rising instances of obesity are causing an explosion of diabetes cases globally.




Comments